The glymphatic system, brain stiffness and the diverse role of neurofluids in brain health.
How neurofluids help the brain to clean itself, allowing it to heal.
We’ve been studying the brain for decades, yet many mysteries remain. What we’re beginning to understand, though, is that long-term brain health depends on more than just neurons firing and synapses connecting. Whirring quietly in the background is a remarkably complex fluid system - clearing out waste, regulating pressure, and keeping the brain’s internal environment in delicate balance.
We now refer to this clean-up and maintenance process as the glymphatic system. For years, it was overlooked, but it’s finally starting to get the attention it deserves - alongside a growing appreciation for the role that fluid movement plays in both healthy brain function and disease.
I’ve mentioned this in passing in a few other posts, but haven’t really gone into depth about how we can take full advantage of it - enhancing the brain’s waste clearance potential to eliminate toxins, optimise brain health and fight cancer.
It’s remarkable that it’s taken us this long to notice really, because when you step back to look at the bigger picture, the implications are huge.
You soon begin to realise that this isn’t just about routine maintenance; disrupted fluid flow is connected to a whole range of common neurological issues - from Alzheimer’s disease and other neurodegenerative conditions, to persistent inflammation, and even the way aggressive brain tumours spread.
For more general health we can transpose onto this ‘milder’, usually more transient conditions, such as intermittent headaches, brain fog and our ability to effectively clear environmental toxins we breathe in day-to-day.
Illuminating new perspectives with emerging technologies
It’s actually possible to measure the effects of this now, thanks to advanced imaging technologies like magnetic resonance elastography (MRE) - a type of MRI many people have likely never heard of, especially when it comes to the brain - and it could provide great value in understanding how the brain adapts, breaks down, or struggles under pressure.
More specifically, we are able to more effectively measure the mechanical properties of living brain tissue - how stiff or soft it is, how it absorbs pressure, and how those properties change with disease.
Traditionally, this type of MRI has been used to assess liver fibrosis, so its potential in brain health is only just beginning to be realised.
MRE provides value beyond traditional imaging modalities
By measuring things like brain stiffness and viscosity, MRE is able to detect changes that other scans might miss - even in areas deep within the brain. It’s still mostly used in research, but one of the first ‘real-world’ uses will likely be pre-surgical planning, helping doctors understand what they’re dealing with before they go in to operate.
It would be a great shame if these applications didn’t extend further, as research suggests that brain MRE could revolutionise how we detect and manage a host of complex neurological conditions - not just by focusing solely on brain cells, but by understanding the environment they live in.
Where I live in the UK, unfortunately MRE is not widely available, especially for brain imaging. Most private imaging centres only offer standard MRI scans (with a handful of exceptions), but do not list MRE as part of their services. To access MRE, you would have to contact research institutions directly, such as CRUK London, who run specific trials and research studies with the technology for brain disorders. I will be investigating this further in future.
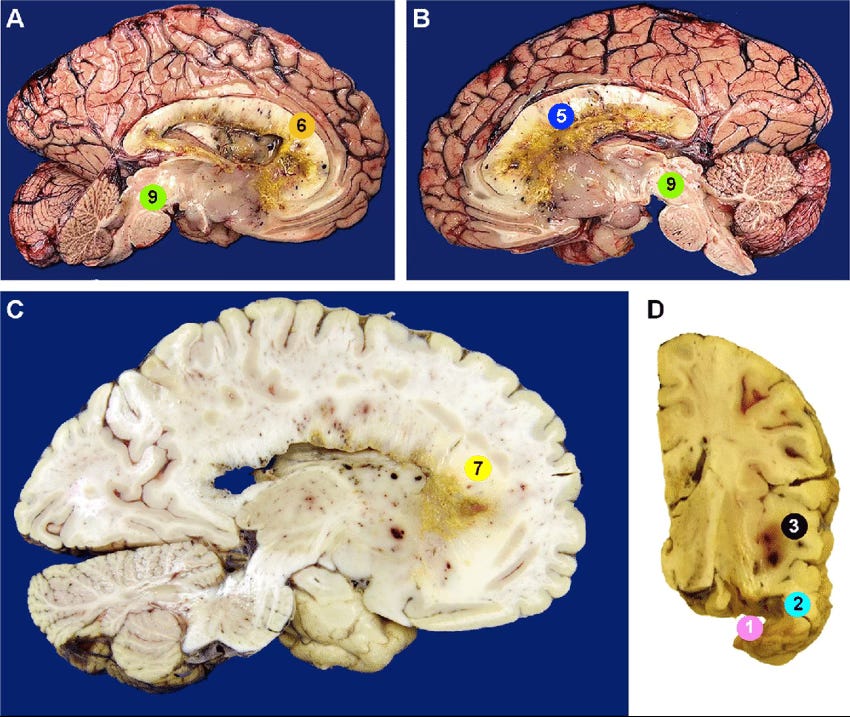
I have a tendency to like to look at the brain as a whole when someone has localised disease, not just the area where the tumour is/was (and surrounding margins), so being able to visualise how something like a brain tumour is disrupting fluid dynamics in the brain could be incredibly valuable. This is not just theoretical, as we already know that tumours (particularly high grade) typicaly affect ‘brain stiffness’ and fluid dynamics beyond their immediate boundaries. In high grade brain tumours the effects can be quite profound and I was always encouraged at how healthy the rest of my brain looked using standard MRI imaging. - though intrigued to explore this further with the aid of more sophisticated tools.
We could even potentially make hypotheses as to how best to treat the brain if we can spot what’s going wrong. This is especially important if the patient decides to undergo treatments like chemotherapy and radiation, because with MRE you should be able to visualise brain toxicity in real-time (or at least how it’s adapting/being shaped).
Practical applications of MRE and its rationale in the monitoring and treatment of brain cancer
Although technically, MRE doesn't directly visualise fluid per se (like CSF or interstitial fluid), we know that it measures the mechanical properties of tissue that are influenced by fluid movement, pressure gradients, and tissue integrity.
Oncologists could easily combine MRE with clinical and biological data. That way, by monitoring changes in brain stiffness, for instance, it is likely that this would correlate with treatment response or side effects - helping to tailor therapies and even flag early signs of relapse, inflammation, or neurotoxicity. For example, radiation-induced leukoencephalopathy has been shown to result in tissue softening, which MRE is well-suited to detect.
The case for implementation of MRE in standard practice
I would argue that radiographers and oncologists should be focusing more on brain stiffness and how efficient each patients brain is at maintaining a healthy state as part of routine imaging. Just looking to see if a tumour has popped up and then reacting to that is a far from ideal scenario, so anything we can do to prevent that should be tested. I would like us to be more proactive and less reactive in everything we do.
We can take advantage of this technology further by adding different MRI techniques like diffusion tensor imaging (DTI) and arterial spin labelling (ASL), which can be combined with MRE to study perfusion, interstitial flow, and glymphatic dynamics with sufficient detail.
Perhaps somewhat controversially, it appears MRE may aid reliable brain tumour diagnosis without the use of contrast agents (see clear images below) and could even help to understand and treat gadolinium retention (or retention of other undesirable contaminants) in the brain.
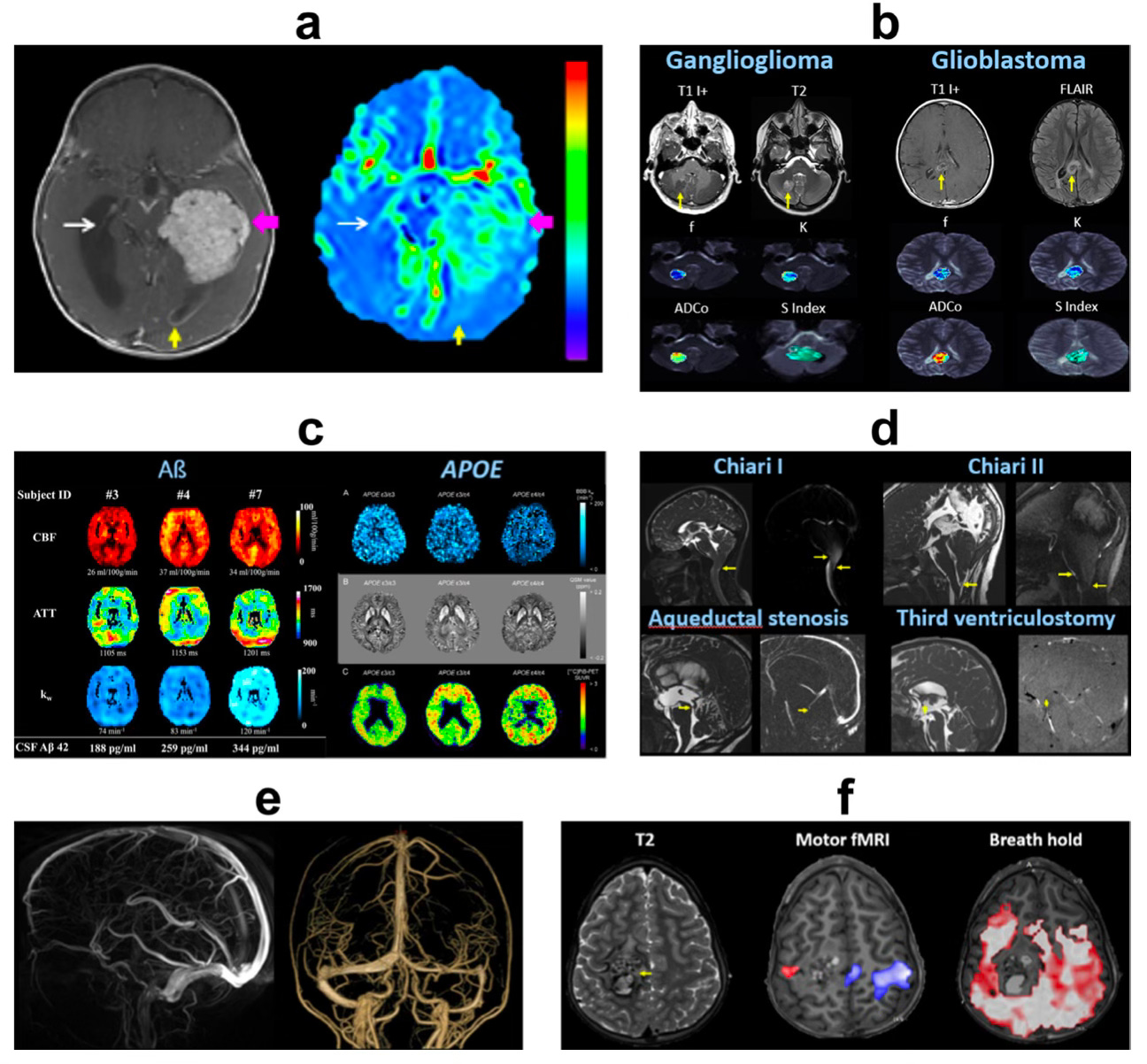
Given that we now know contaminants like metals and micro plastics can be retained in brain tissue, and that even the type of diet patients follow could potentially alter the risk of toxicity to contrast agents, it is within everyone’s best interest to make the most of this technology.
Unfortunately most of us will be exposed to carcinogens and volatile organic compounds in our daily lives and we don’t want that to contribute to or cause serious disease as they accumulate in tissues. It makes sense to do everything possible to maintain robust detoxification systems in the body, which includes the brain, and be able to quantify how efficient we are at achieving this.
Knowledge evolves with time
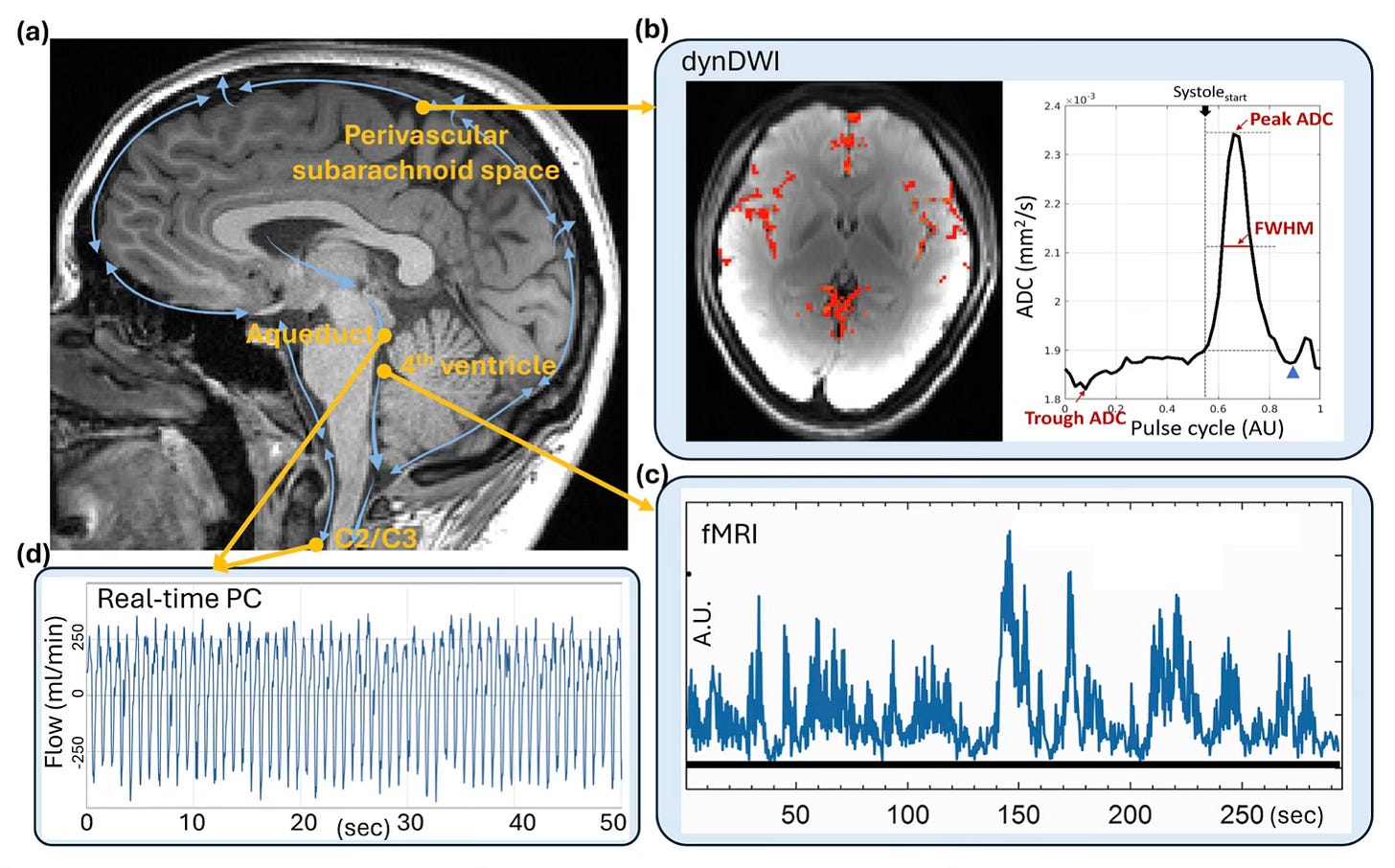
Fluid forces in the brain are not passive. Not so long ago, we still believed that cerebrospinal fluid (CSF) mainly played a passive role - that is was just there to cushion the brain and stop it from knocking against the skull… but that view is now seriously outdated.
Thanks to advances in real-time MRI, we know that CSF - and the interstitial fluid (ISF) that surrounds brain cells - moves in rhythmic, wave-like pulses. These movements aren’t random either; they’re closely tied to our heartbeat and, perhaps even more importantly, our breathing. - as is beautifully illustrated in the images below.
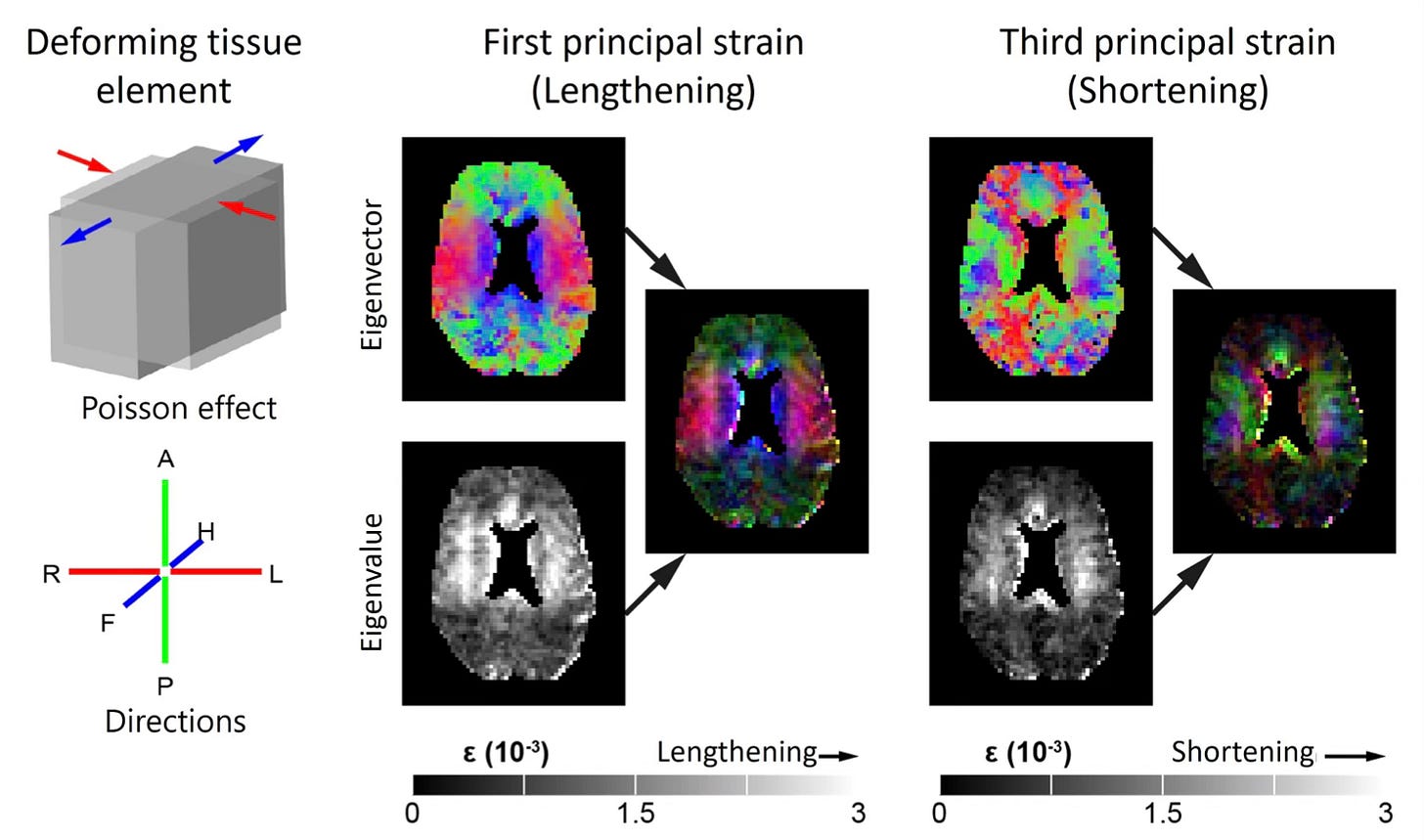
How we breathe actually plays a big part in how these fluids move. Deep breaths, especially, cause shifts in pressure in the chest that help pull CSF upward into the brain. These flows aren’t just background noise - they help clear metabolic waste, balance pressure, and maintain the right chemical environment for neurons to function properly.
Take Alzheimer’s disease, for example. A major problem in this condition is the buildup of amyloid-β and tau proteins. These are normally cleared out by fluid exchange systems like the glymphatic pathway. While most of the strong evidence for this comes from animal studies, new imaging data in humans is beginning to support the idea that when CSF–ISF flow is impaired, these harmful proteins start to accumulate.
We can argue whether this is a chicken or egg situation and what this means from a purely metabolic perspective, but here I’m just focused on how we visualise the phenomena. - and Alzheimer’s provides a perfect model for this.
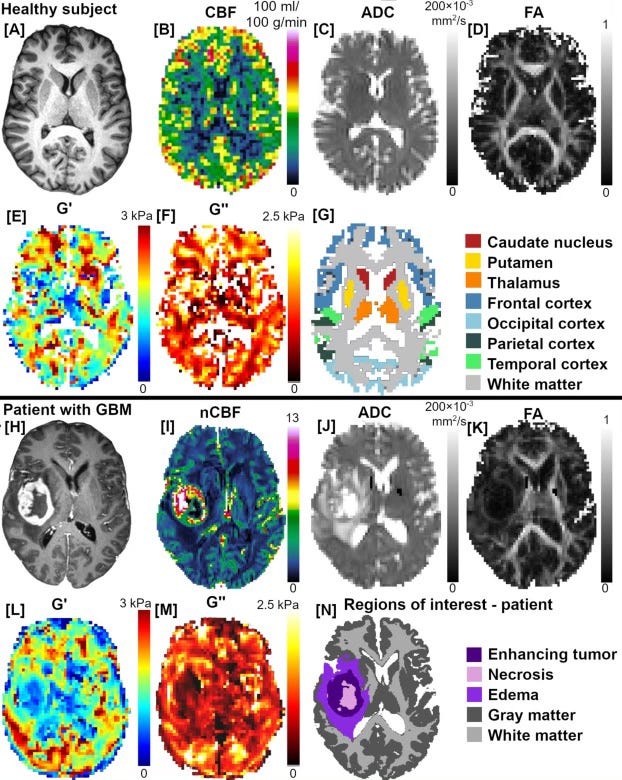
In gliomas, the story becomes every more complex and interesting. Tumours can physically disrupt fluid movement by pressing on tiny spaces around blood vessels and scrambling the brain’s structure. As you can imagine, the effects can be wide ranging.
This kind of fluid stagnation likely does more than just block waste clearance
Keep reading with a 7-day free trial
Subscribe to Andrew’s Substack to keep reading this post and get 7 days of free access to the full post archives.