The next big thing? - Questions and answers about IDH inhibitors
There is renewed interest in IDH inhibitors, as new drug Voranigo is granted FDA approval. Is the hype justified?
Voranigo (active ingredient vorasidenib citrate) is a new drug receiving significant attention following encouraging Phase 3 clinical trial data as part of the INDIGO trial. It is the most recent IDH inhibitor to gain FDA approval and has now been reported widely in the press among various news outlets and brain tumour organisations.
The potential implications following results of this trial is certainly not trivial - as is too often the case with these hyped up ‘breakthrough’ drugs. There are metabolic consequences to the IDH mutation that have broad metabolic effects.
The drug has a particular benefit in the treatment of low grade gliomas harbouring isocitrate dehydrogenase (IDH) mutations, oligodendroglioma and higher grade IDH mutant brain tumours that have arisen from lower grade tumours.

A slew of questions naturally arise when a promising new drug comes to the fore, especially one that generates so many headline grabbing quotes and soundbites. I hope to address at least some of these questions.
I have often felt that reporting on new developments in the brain tumour world is typically unbalanced, unrealistic and skewed too much in favour of a certain narrative that is overly optimistic. This is understandable perhaps, given that the message is usually relayed from big pharmaceutical companies to ill informed journalists who need a good news story that will generate clicks as part of our 24 hour news culture.
With that being said, I have greater hope for IDH inhibitors than for example, anti-angiogenic therapies, which have so far been underwhelming. Apparent improvements in progression free survival have not translated to a benefit in overall survival for many of these drugs.
I feel genuinely optimistic about the promise of Voranigo and would echo the sentiments of others who believe it could represent a pivotal moment in how we treat brain tumours, even beyond the type it is currently indicated for.
My train of thought following the news about Voranigo led me to ask myself the following questions…
Q1. What is the significance of IDH mutations in brain cancer and how does Voranigo work?
To answer this question fully, we need to take a step back to look at the bigger picture before zooming in on the details.
In the past I have written about how IDH (Isocitrate Dehydrogenase) mutations, IDH1 and IDH2 in particular, are common in various types of brain cancer. These mutations not only help classify tumours, but also provide useful information on survival, treatment resistance, and in some highly compelling cases, serve as a target for reducing seizure frequency.
While IDH1 and IDH2 are the primary enzymes of interest, there is also a third enzyme, IDH3, which plays a role in cellular metabolism and potentially in cancer development, but it does not typically mutate in brain cancer—I will touch on this later, as it is worth mentioning.
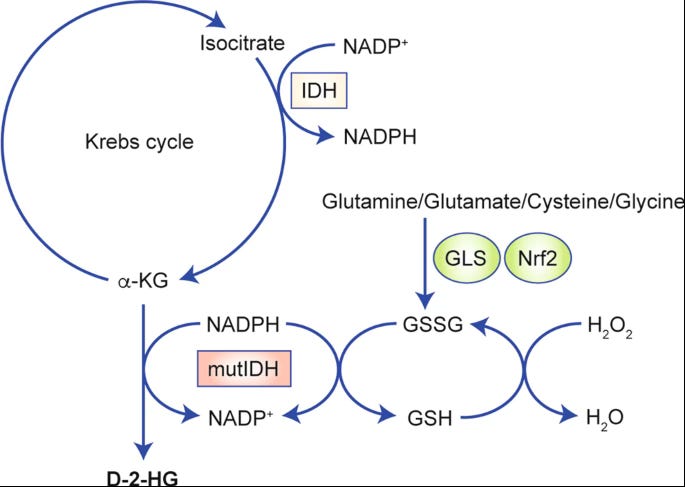
The process is quite complex, but essentially, when either IDH1 or IDH2 (or both) mutate, they produce an abnormal metabolite called D-2-hydroxyglutarate (D2HG). Under normal circumstances, these enzymes support regular cellular functions, but when mutated, they generate D2HG instead. This accumulation of D2HG disrupts normal cellular processes, promoting tumour growth and increasing the cancer's aggressiveness.
* Update - Just for clarification, I thought I would add the following commentary for those that may be confused about ‘good’ and ‘bad’ mutations. Upon sharing this article, I received a question regarding the apparent contradiction between the accumulation of D2HG in IDH mutant brain tumours, which increases cancer aggressivity and promotes tumour growth, and the fact that this mutation is traditionally considered favourable. This is an excellent question, and I realise I should have provided more explanation initially. I hope my response below helps clear up any confusion.
Some background
With so much information at our fingertips on the IDH mutation, you may be forgiven for thinking we’ve understood this for many years, when in fact, that is not the case at all. Our understanding of IDH mutations and their role in cancer development is a recent discovery, with significant breakthroughs occurring around 2008-2009. As such, IDH inhibitors are relatively new in oncology, especially for brain cancer, and our understanding continues to evolve. We’ve come a long way in a short space of time.
To date, Ivosidenib (Tibsovo) has been the only IDH inhibitor that has been explored specifically for brain tumours, and this is only a recent development. Ivosidenib has been investigated in clinical trials for its potential to treat patients with recurrent or progressive IDH1-mutant gliomas. It still isn’t widely approved for brain tumours, having been traditionally used primarily for other cancers, but it’s all we had until now. That’s why FDA approval of new drug Voranigo is such a major development.
We have now reached a point in time where data from human trials supports preclinical models and mechanistic observations for the use of an IDH inhibitor designed specifically for brain cancer, with studies suggesting that new drug Voranigo is able to directly target the production of D-2HG, potentially slowing tumour growth and progression of different types of brain tumour.
Q2. Considering the fact that IDH inhibitors are not new - why is there so much hype surrounding FDA approval of this new drug?
The best way to approach this question is probably to start with answering some basic questions about Voranigo and seeing what we can learn from results of the INDIGO trial.
Firstly, we know that the drug effectively crosses the blood brain barrier without harming healthy cells and side effects are relatively mild. That is the overwhelming benefit. Although IDH inhibitors used in other cancers have shown some benefit in brain cancer, they have not proven to be as effective as Voranigo in Phase 3 clinical trials.

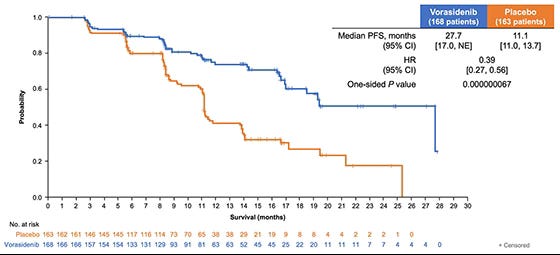
Furthermore, reports indicate that Voranigo has been shown to achieve adequate levels of the drug in the central nervous system, suggesting it should be highly effective against brain tumours. We know that in the Indigo trial, Voranigo significantly extended progression-free survival (PFS) compared to placebo - as shown below - and that patients taking Voranigo showed a median PFS that was nearly double that of the placebo group.
Mechanistically, Voranigo is a dual inhibitor, meaning is inhibits both IDH1 and IDH2 enzymes. These mutations are common in low-grade gliomas, particularly in diffuse astrocytomas and oligodendroglioma. That doesn’t mean to say it won’t be effective for other types of brain tumour, or even some CNS conditions and secondary brain cancers, but it was specifically designed for this scenario.
As a treatment for low grade gliomas (the drug’s primary indication), this treatment is unique. Current therapies, such as Temozolomide or radiation therapy, are highly toxic, less targeted, and come with significant side effects, including cognitive decline and the risk of secondary cancers (not exclusively in the brain). Long term use of Temozolomide has even been linked with a higher risk of developing leukaemia in some cases. It’s rare, but it happens, and the risk increases the longer you’re on it. Voranigo is taken orally and has a much more favourable side-effect profile, making it more convenient and far less toxic.
I have often said that the ‘watch and wait’ approach is morally questionable, because it is reactive and not proactive. Not having any other options is no excuse for not pursuing a more proactive approach with non toxic therapies. Voranigo therefore provides us with a unique opportunity to intervene, with the first FDA-approved therapy specifically targeting IDH-mutant low-grade gliomas.
The ability of Voranigo to significantly prolong progression-free survival in a population with limited treatment options is a major advancement. It’ll be interesting to see if this result can be replicated on a larger scale. That isn’t often the case, only time will tell, but the prospect is certainly intriguing. There is also great potential for the use of Voranigo with metabolic therapies to enhance its effectiveness further, given that glucose availability is a major driver of the accumulation of the resulting metabolites.
Now that we have laid the foundations, let’s go a bit deeper to answer a few more specific questions about IDH mutations and brain tumours.
Q3. How might an IDH inhibitor improve seizure threshold?
Generally speaking, we know that seizures are common in glioma patients due to the excitotoxic environment created by the tumour.
Interestingly, it is often the case that seizures are more frequent in lower-grade brain tumours compared to those of a higher-grade. This is largely due to the fact that we see less neuronal death and structural disruption in these tumours. As a result, the neurons in the affected area remain relatively functional but are hyperexcitable, leading to an increased likelihood of seizures. Please note that I am referring to the frequency of these seizures, not necessarily their severity, and so I am not saying that individuals with more aggressive disease don’t experience them with the same type of devastation.
The accumulation of 2-HG (that we see with IDH mutant glioma brain tumours) contributes to this neuronal hyper-excitability by altering cellular metabolism, increasing oxidative stress and influencing neurotransmitter activity. Elevated levels of 2-HG can therefore provide us with a direct indicator of the likelihood of seizures for these individuals, and can be measured with sophisticated MRI imaging - at and around the tumour area for information on metabolites using magnetic resonance spectroscopy and other diagnostic tools. I briefly touched on some of these advanced imaging modalities in my summary of this year’s BNOS conference.
In recent years there have been some compelling case studies of seizure resolution in brain tumour patients with drug resistant epilepsy using IDH1 inhibitors for oligodrendroglioma, and we understand the broad mechanisms of seizure development for IDH mutations in other types of brain tumour.
Now that we have a dual inhibitor that appears to show greater efficacy to treat brain tumours, it would be interesting to see if we can achieve similar results across a broader range of individuals with different types of brain tumour.
Q4. How can I access these drugs and find out if I’m eligible?
Clues on eligibility will be in the histopathology report, which I encourage all patients to receive a copy of post diagnosis. There you will see IDH1 and IDH2 status as common genetic indicators of treatment susceptibility and prognosis. In my specific case, I was IDH1 wild type, which is traditionally indicative of a poorer prognosis, however IDH2 was shown to be mutated. IDH1 inhibitors therefore would be of little interest to me, though Voranigo is a dual inhibitor, which makes it appealing.
While typically, IDH1 mutations are more common, and the majority of gliomas with IDH mutations involve IDH1, if IDH2 is mutated and IDH1 is wild type, it still indicates a similar underlying biological mechanism. I would always argue that you should look at both if IDH1 is wild type for greater clarity. - especially keeping in mind that we now have access to dual inhibitors.
You may be asking how IDH1 and IDH2 differ, and this is important to briefly outline.
Put simply, IDH1 and IDH2 mutations affect different parts of the cell. IDH1 is primarily found in the cytoplasm, the fluid part of the cell outside the nucleus, where it helps with cellular metabolism. IDH2, on the other hand, is located in the mitochondria, the cell's energy-producing structures. Both mutations lead to the production of the same harmful oncometabolite, 2-HG, just in different locations.

If you have been able to identify a potential benefit in taking these drugs, it is always best to speak to your oncologist. Personally, I would advise going with a study or some compelling evidence in support of Voranigo for your individual case. In the UK, if the drug isn’t available on the NHS, you may either have to see if private medical insurance will cover it, or if there are clinical trials you can join.
I would recommend checking the clinical trials database myTomorrows for suitable clinical trials on dual IDH inhibitors and receiving a referral from your oncologist through the user friendly online portal.
Q5. What about side effects?
The most common side effects observed in clinical trials of Voranigo include elevated liver enzymes (transaminase increases), fatigue, nausea and diarrhoea. These relatively mild and reversible side effects can all be managed with regular monitoring and adjustment of medication.
Monitoring for side effects would include liver function tests (liver enzymes and bilirubin), cardiac monitoring (QT intervals via ECG) and general symptom monitoring (reporting of new or worsening symptoms - fatigue, GI issues, cognitive changes etc.).

Q6. How might ketogenic metabolic therapy (KMT) influence this? Are there any additional considerations?
Therapeutic ketosis and ketogenic therapies have several mechanisms of action that could be beneficial here beyond simply ‘starving the tumour of glucose’.
Keep reading with a 7-day free trial
Subscribe to Andrew’s Substack to keep reading this post and get 7 days of free access to the full post archives.