The side of the story you don't hear
Ipilimumab is an exciting new treatment for high grade glioma, but the media isn't covering the whole story.
This week, I will share my thoughts on why the immunotherapy drug Ipilimumab, despite showing promising responses in several cancers since its FDA approval in 2011, has seen stalled progress in clinical trials for brain cancer, even with some truly remarkable individual case studies.
Quite rightly, the media has shown great enthusiasm for these isolated successes, but journalists fail to appreciate the whole story. In the chase for clicks behind paywalled content - as most of these articles on the subject are - the appreciation of important context and nuance goes missing, and sensationalist headlines don’t always align with the facts.
2019 was expected to be a big year, acting as a springboard for advancing research on using Ipilimumab to treat brain cancer in clinical settings.
Funded by The National Brain Appeal, the Ipi-Glio trial, led by consultant neuro-oncologist Dr Paul Mulholland, became the largest-ever immunotherapy trial for brain cancer. A total of 119 patients were recruited to assess whether Ipilimumab, administered after the standard of care (surgery, if possible, followed by radiotherapy and chemotherapy), could improve patient outcomes.
Encouraging results from the trial led to a follow-on study, the NeAT Glioma trial, in which Ipilimumab would be given to glioblastoma patients before the standard treatment.
Since there are very few examples of such an approach in these kinds of studies, I personally found this trial design encouraging, and I’m sure many others would too. It would have been interesting to see the effects of the drug before any other intervention.
I believe it's important to stay open-minded, innovation is key. If you do what you’ve always done, you’ll get what you’ve always got. We all hope for a future where we no longer have to rely on toxic, destructive therapies that are either ineffective or offer only marginal benefits.

There was great hope and anticipation for the NeAT Glioma trial, especially given the encouraging results of the first trial and the novel design of this one. Considering the high number of patients recruited in the initial Ipi-Glio trial and the clear demand for this treatment, it may seem surprising that the NeAT Glioma trial had to close due to a lack of patient recruitment.

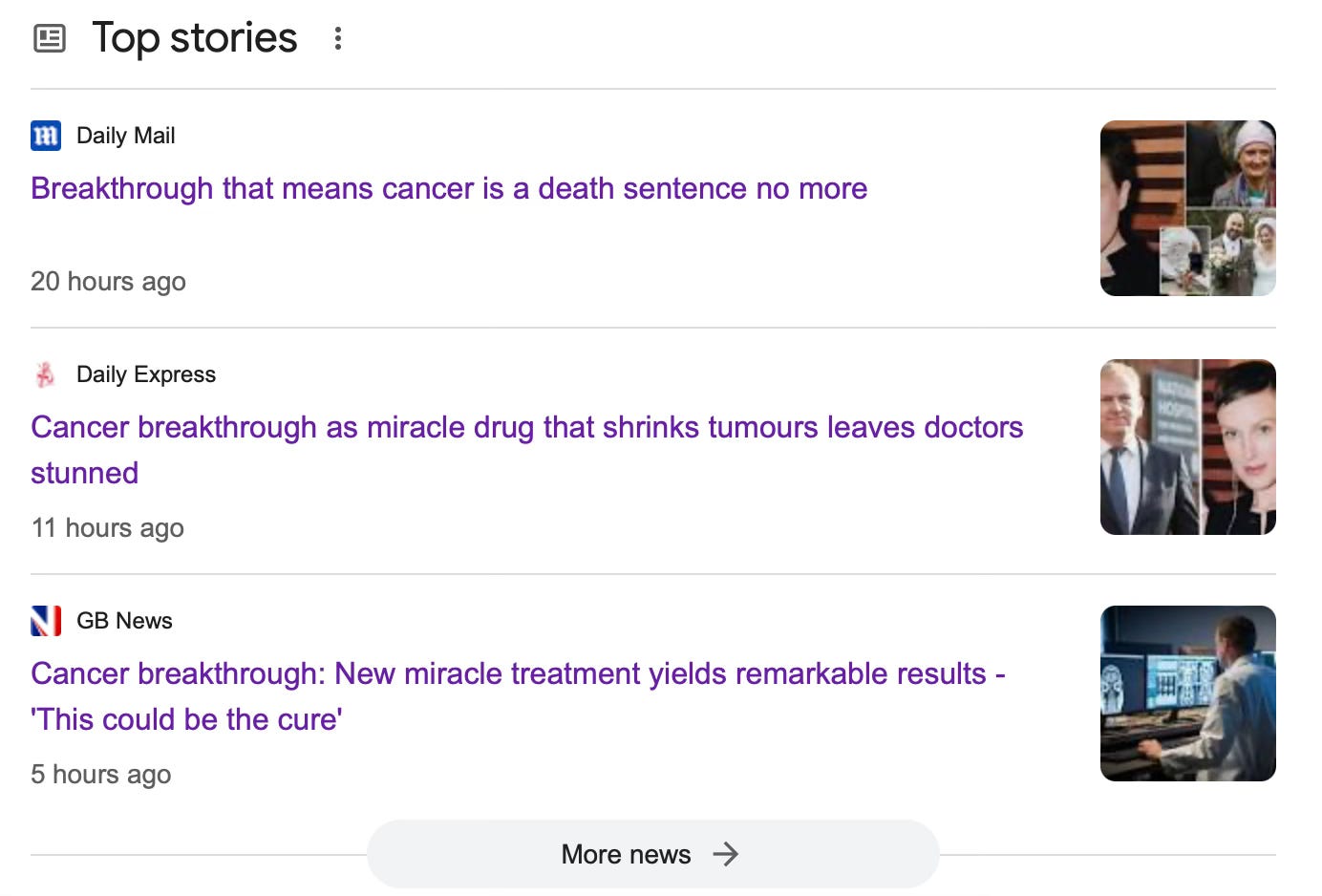
Despite the disappointing closure of the trial due to a lack of participants, Ben Trotman, 41, the only glioblastoma patient recruited, is doing well 16 months after treatment - his tumour shrank and he is exceeding his projected life expectancy. Ben began treatment in November 2022 and is believed to be the first person in the world to receive immunotherapy for glioblastoma before the standard of care.
This raises inevitable questions about why the NeAT Glioma trial struggled with patient recruitment, especially given the success of the initial Ipi-Glio trial - and the encouraging treatment responses of other patients outside of the trial.

It is likely that the trial faced recruitment challenges due to stricter eligibility criteria, as it focused on administering immunotherapy before standard treatments, an innovative but ‘less proven’ approach that may have caused hesitancy among clinicians. Then we have other potential factors, such as limited patient awareness, logistical barriers and competition from other trials or established treatments. A perfect storm that could have further contributed to the difficulty in attracting participants, despite the initial success of the Ipi-Glio trial.

In my next post, I will provide more information on early trial results, more specific reasoning behind the cancellation of the next trial phase, a balanced critique of the drug’s success stories, its mechanism of action and guidance for patients interested in learning more about its accessibility, side effects, and suitability for their condition.
You won’t get this from reading any of the articles online through the mainstream media. Important context and detail is omitted, which is not helpful for patients seeking answers.
I remain optimistic about this treatment, the issue lies in access, how we fund it, and how it is studied. We already have three high profile cases to examine and investigations at different institutions across the world, under different clinical circumstances. This includes 2 patients in the UK with high grade glioma brain tumours (anaplastic astrocytoma and glioblastoma) and a scientist in Australia with glioblastoma, who has been documenting his progress on social media. Advancement often comes through advocacy and social media has become a powerful tool.
Finally, I will be covering what the likely future of this therapy looks like, how organisations such as The National Brain Appeal are integral to it’s advancement, and I will follow this by explaining how individuals such as Dame Siobhain McDonagh and Dr Paul Mulholland are influencing how we can recruit patients, gain access to these types of treatment and ensure they are successful through their tireless work, compassion and advocacy for better options to treat brain cancer.




Dear Andrew
Would you be willing to do a personal call with a brain cancer patient from India? We wanted to discuss about diet and other protocols you suggest
Is it right that Richard Scolyer has no evidence now of glioblastoma? I’ve never read this in this Facebook posts and he doesn’t seem to respond to any questions on Facebook posts or private messages. It seems you do have extra info which would be very useful to me.
You will also be glad to know that you avoided having me nagging for info from you when you couldn’t make it to the Manchester Ketobrainhealth seminar. 🤣🤣🤣